Overview
NCI-funded researchers – intramural and extramural – are encouraged to share their data through the CRDC, in line with NIH’s Data Management and Sharing Policy. The CRDC Submission Portal supports researchers by facilitating the process of submitting data to the CRDC, which includes two distinct steps:
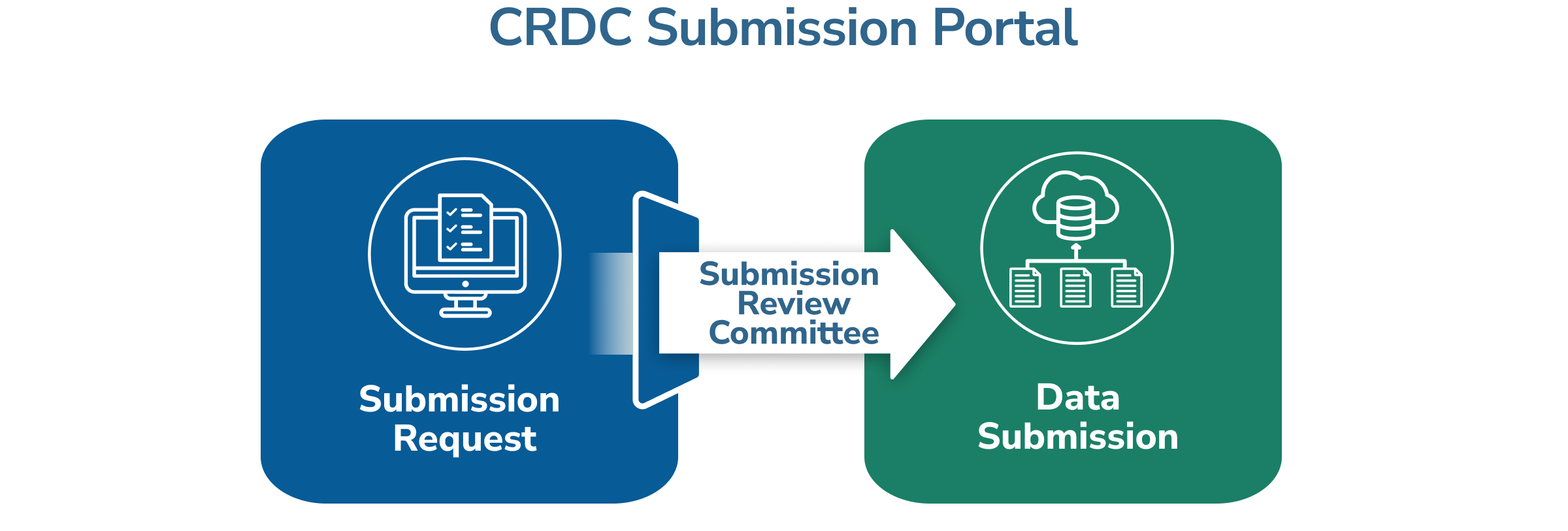
- Submission Request: This involves completing an application form in the CRDC Submission Portal that is reviewed by the CRDC Submission Review Committee. Download the Submission Request Instructions.
- Data Submission: This involves uploading data through the CRDC Submission Portal. This process is open once the Submission Request has been accepted by the CRDC Submission Review Committee. Download the Data Submission Instructions.
Researchers are advised to allow sufficient time for the full process to be sure they comply with the data sharing timelines specified by the NIH Data Management and Sharing Policy.
CRDC Requirements
The CRDC Submission Review Committee reviews Submission Request forms and considers how the data and the study methodology contribute to the greater research community. The team also considers if:
- Studies are NCI-funded or NIH-funded
- Study data collection is complete and data are ready to be submitted
- The study has been registered in dbGaP, if it contains controlled access data
- The data are from human, canine, mouse, zebrafish, or other studies
The submitter will be requested to confirm that the data they plan to submit are de-identified.
For all NIH-funded data, the PI/research team must have the appropriate authority to submit data to the CRDC. The research team must ensure the submitted information is in accord with the original study consent, all applicable laws, regulations, and institutional policies. CRDC is not able to help interpret consent forms. If there are any questions about appropriate permissions to submit, the research team should consult with their Institutional Review Board (IRB) to validate the authority to submit.
Submission Request
Process and Timing
The Principal Investigator (PI) or the primary contact will fill out the Submission Request form through the CRDC Submission Portal. This is where the researchers have the opportunity to provide details about the study data they intend to submit. Once completed, the CRDC Submission Review Committee will evaluate the application and share their decision in four to six weeks. The status of the request can be tracked through the Submission Portal.
Note: Studies that contain controlled access data must be registered in dbGaP prior to completing the Submission Request form. Learn more about registering your study in dbGaP.
Instructions and Portal
Submission Request Instructions Detailed instructions are provided as a PDF.
CRDC Submission Portal Link to the portal to start the Submission Request form.
Data Submission
Process and Timing
Once the Submission Request has been approved by the CRDC Submission Review Committee, the submitter may proceed to data submission. The PI or primary contact will be assigned a data concierge who will work with them and provide assistance throughout the data submission process.
The submission process involves uploading and validating a metadata manifest and the data files through the CRDC Submission Portal. When a dataset has passed all validations, the final submission is pushed to the Data Submission team for review before it is released to the appropriate CRDC Data Commons, which make the data available through their portals.
For users looking to align their data with CRDC standards before starting the submission process, the data model viewer is available to outline the types of data required. Users can also download the data dictionary and sample metadata templates to guide their submission process. These resources are available through the CRDC Data Submission Portal through the Model Navigator in the menu.
In addition, a comprehensive list of CRDC standard CDEs can be found at caDSR. Click the “CRDC Standard Data Elements” link in the Links to Favorites section or download them from the getCRDCList endpoint of the caDSR API.
Instructions and Portal
Data Submission Instructions Detailed instructions are provided as a PDF.
CRDC Submission Portal Link to the portal to start the Data Submission.
Post-Submission Considerations
Once a submission is complete and data are released on a CRDC data commons, a researcher – or a designated member of the research team that submitted the data – can log in to the CRDC Submission Portal and use the Data Explorer to access the metadata for the submitted dataset. They can review and download this metadata to identify and guide any necessary corrections to the hosted data. The metadata can also be reused to support future submissions with similar information.
Instructions and Portal
To access the Data Explorer, users must log in to the CRDC Submission Portal with the same credentials and passcode used for the original data submission.
Data Explorer InstructionsDetailed instructions are provided as a PDF.
CRDC Submission Portal Link to the portal to access the Data Explorer.
Helpful Links
In submitting data to the CRDC, users are expected to understand NIH’s Data Management and Sharing Policy, general procedures for working within the NIH/NCI data ecosystem, and CRDC’s data standards.
- FAQs - Find answers to common questions
- Learn more about establishing a Login.gov account
- Learn more about NIH’s Data Management and Sharing Policy
- Learn more about NIH's Common Data Elements (CDE) guidelines
- Learn more about CRDC’s Data Commons
- Learn more about the Select Datasets housed within the CRDC